What keeps you up at night?
For those treating IPF Patients, it could be…
A nightmare called EXACERBATIONS
Acute IPF exacerbations are rare but devastating events that significantly impact patient prognosis1-5
Estimated 5% to 10% annual incidence of Acute IPF Exacerbations3










Acute ipf exacerbations significantly impact survival3
Median survival in patients with IPF after an Acute IPF Exacerbations is ~3-4 months5
50% of patients hospitalised for an Acute IPF Exacerbations die during Hospitalisation4
So who are at risk?
Acute IPF Exacerbations are a risk to all patients with IPF because they may occur at any time during the course of the disease without warning or known cause3
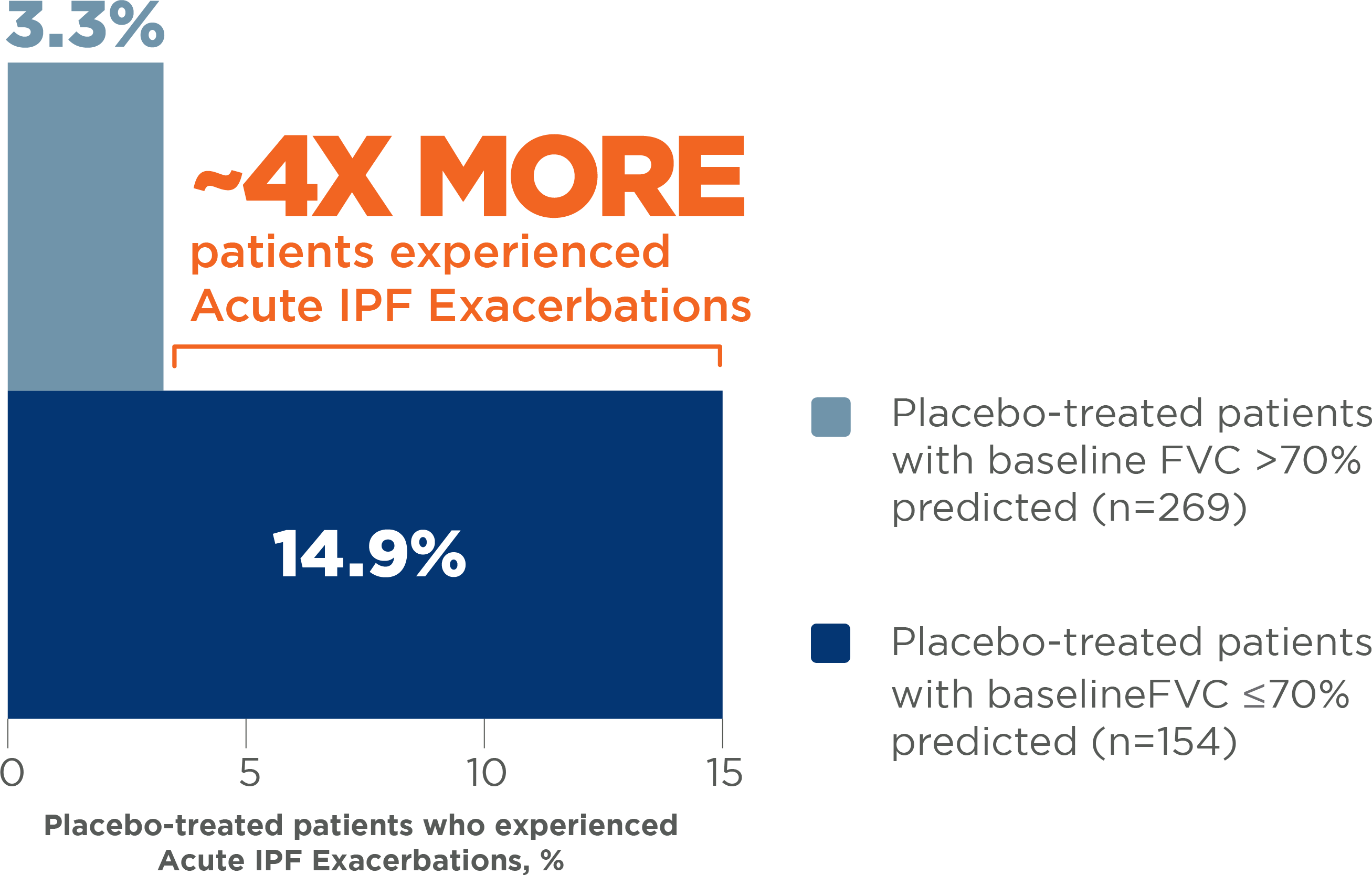
Patients with more severe impairment in FVC are at greater risk for an Acute IPF Exacerbation7
Placebo-treated patients with baseline FVC ≤70% predicted had a higher incidence of Acute IPF Exacerbations than patients with baseline FVC >70% predicted 7
What did these acute Exacerbation look like?
Acute Exacerbation of ILD was defined as acute, clinically significant respiratory deterioration characterized by evidence of new, widespread alveolar abnormality meeting these criteria8,9
Acute worsening or development of dyspnea,
typically <1 month duration


Computed tomography with new bilateral ground-glass opacity and/or consolidation superimposed on a background pattern consistent with fibrosing ILD
Deterioration is not fully explained by cardiac
failure or fluid overload

Infection was not an exclusion criterion for an Acute Exacerbation.9
Reduce the risk of A nightmare called Exacerbations
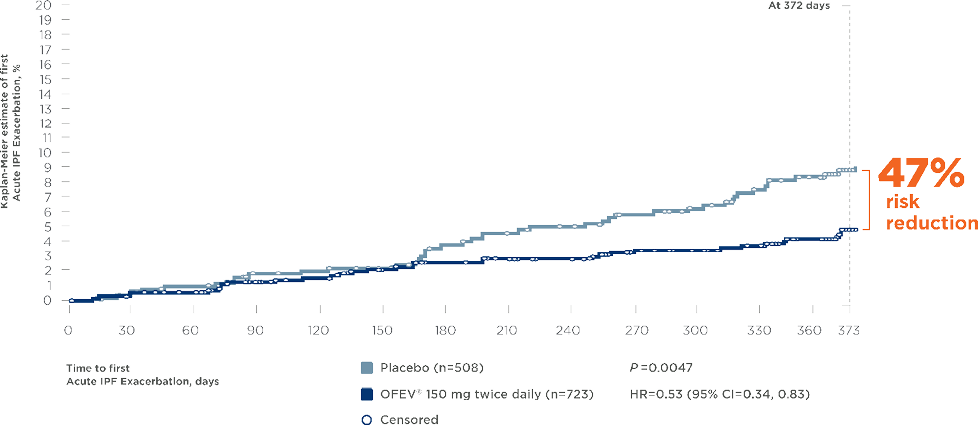
OFEV ® (nintedanib) significantly reduced the risk of Acute Exacerbations in patients with IPF*10, 11
Pooled INPULSIS®-1 and -2 and TOMORROW trials11
- * Diagnostic criteria for acute IPF exacerbations were prespecified in the trial protocol features within 1 month, including all of the following:
- • Unexplained worsening of dyspnea or development within 30 days
- • New diffuse pulmonary infiltrates on chest x-ray, and/or new HRCT parenchymal abnormalities with no pneumothorax or pleural effusion (new ground-glass opacities) since last visit
- • Exclusion of infection as per routine clinical practice and microbiological studies
- • Exclusion of alternative causes as per routine clinical practice and including the following: left heart failure, pulmonary embolism, and identifiable cause of acute lung injury † Pooled analysis of data from three 52-week trials with OFEV®, INPULSIS®-1, INPULSIS®-2, and TOMORROW, including 1231 patients with IPF. Time to first investigator-reported Acute Exacerbation over 52 weeks was a predefined secondary endpoint in the TOMORROW and INPULSIS® trials.
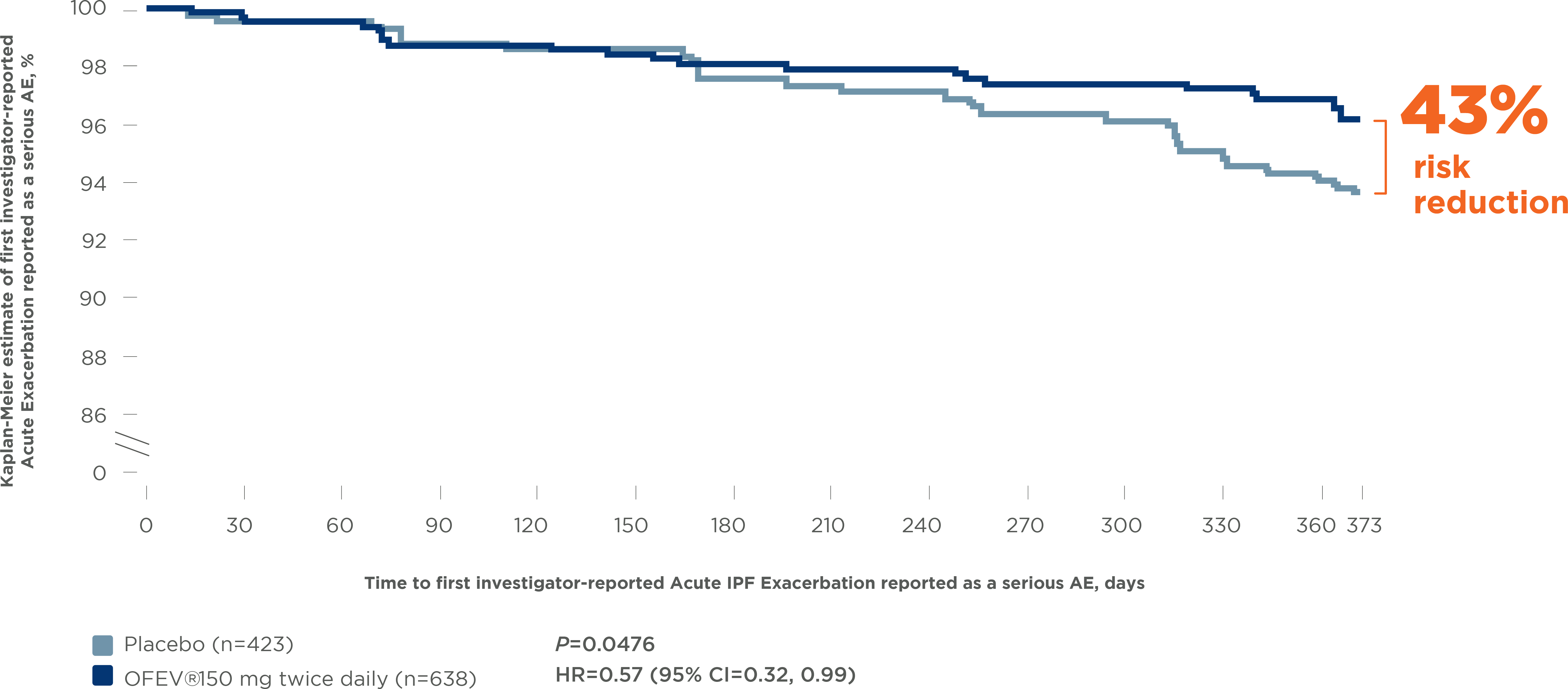
OFEV® significantly reduced the risk of Acute IPF
Exacerbations reported as serious AEs by 43% 12
Pooled INPULSIS® trials: Time to first investigator-reported
Acute IPF Exacerbations reported as a serious AE
AE, adverse event
OFEV®is a recommended treatment for IPF according to
the 2015 ATS/ERS/JRS/ALAT Guidelines13
IPF EFFICACY PROFILE
- OFEV® consistently slows disease progression with an ~50% reduction in FVC decline across a broad range of patient types13
- OFEV® significantly reduces the risk of acute IPF exacerbations by ~50%10,11
- OFEV® has clinical experience exceeding 80,000 patient-years Worldwide14

IPF SAFETY PROFILE
- OFEV® demonstrated a favourable risk-benefit profile 10
- AEs can be effectively managed in most patients 1
- Mild to moderate diarrhea is the most frequent AE and occurred mostly in the first 3 months of treatment15
- While 62% of patients experienced diarrhoea, less than 5% discontinued treatment15
DOSING SIMPLICITY
- Just 1 capsule, twice daily 15
- OFEV® is administered orally in a 150 mg capsule†
- No up-titration required when initiating treatment 15
ALAT, Latin American Thoracic Association; ATS, American Thoracic Society; ERS, European Respiratory Society; FVC, forced vital capacity; IPF, idiopathic pulmonary fibrosis; JRS, Japanese Respiratory Society.
* This conditional recommendation means that clinicians are encouraged to discuss preferences with their patients when making treatment decisions.2
† OFEV® 100 mg capsules are available for AE management and for the treatment of Child-Pugh A patients.15