537 million people
worldwide are living with diabetes. By 2045, an estimated 783 million people worldwide will live with diabetes2
For your patients with type 2 diabetes, CV death may be closer than you think.1
For your type 2 diabetes patients with hypertension and coronary artery disease (CAD) who are on metformin5, the battle against CV death has
already launched9
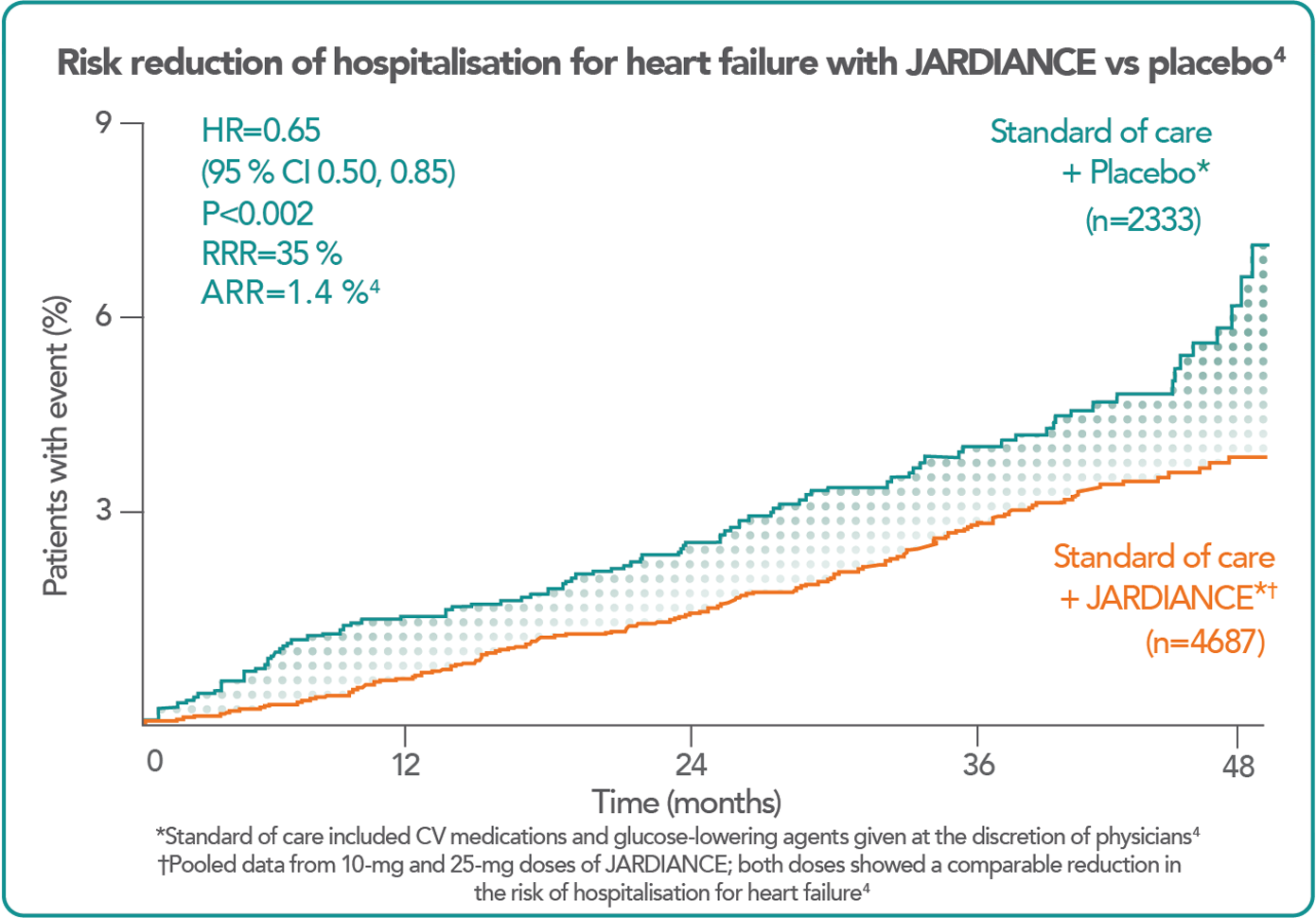
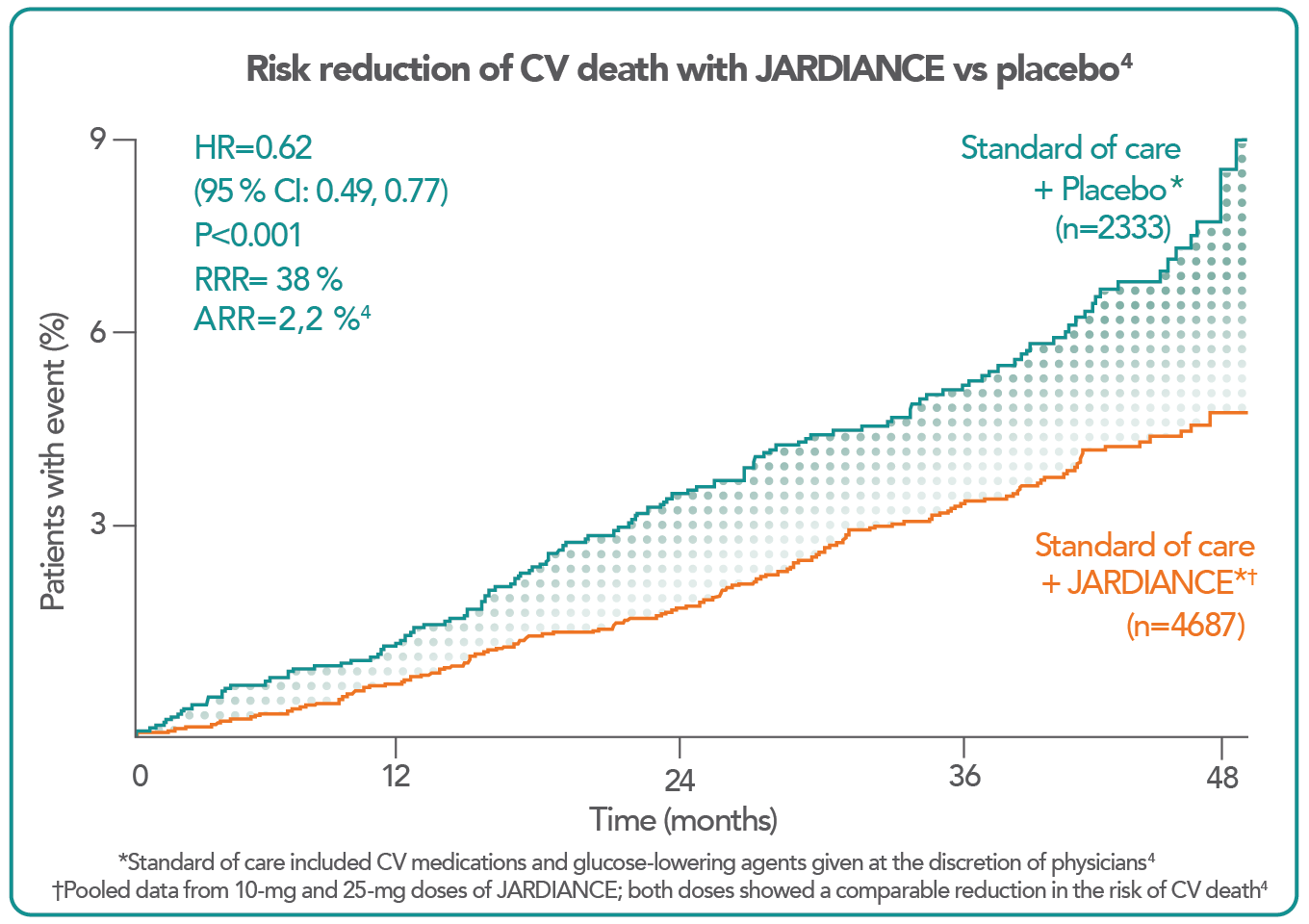
JARDIANCE allows you to do more to protect your patients from the risk of CV death.4
People with type 2 diabetes are
2-4x more likely
To develop cv disease than people
without diabetes1

Despite advances in care
one in two patients
with type 2 diabetes still dies of a
CV event worldwide5




Over 4 MILLION patient-years of treatment8
Over 4 years of worldwide experience8

In the landmark EMPA-REG OUTCOME® trial, in patients with type 2 diabetes and established CV disease – most commonly CAD4
Established CV disease may include:
Vascular manifestations:4,7
- • Coronary artery disease (CAD)
- Evidence of single or multi-vessel disease
- • Peripheral artery disease (PAD)
- or

Prior atherothrombotic events:7
- • History of myocardial infarction (MI)
- • History of stroke
>75 % of patients had arterial vascular disease7
20.8 % of patients had PAD
65 % of patients had a history of MI/and or stroke7

JARDIANCE is the most commonly prescribed SGLT2 inhibitor in the world, and it is now recommended by more than 50 guidelines worldwide.8
The 2019 ADA-EASD Consensus report states:9
- The selection of medication added to metformin is based on the presence of established ASCVD and other comorbidities such as HF or CKD.9
- Select an SGLT2 inhibitor such as Jardiance, with proven CVD benefit for patients with ASCVD with or without HF (in patients with adequate GFR).9
JARDIANCE offers convenient, once-daily oral dosing:3
- The recommended starting dose of JARDIANCE is 10 mg once daily
- In patients tolerating JARDIANCE 10 mg once daily and requiring additional glycemic control, the dose can be increased to 25 mg once daily
- JARDIANCE can be taken with or without food, at any time of the day



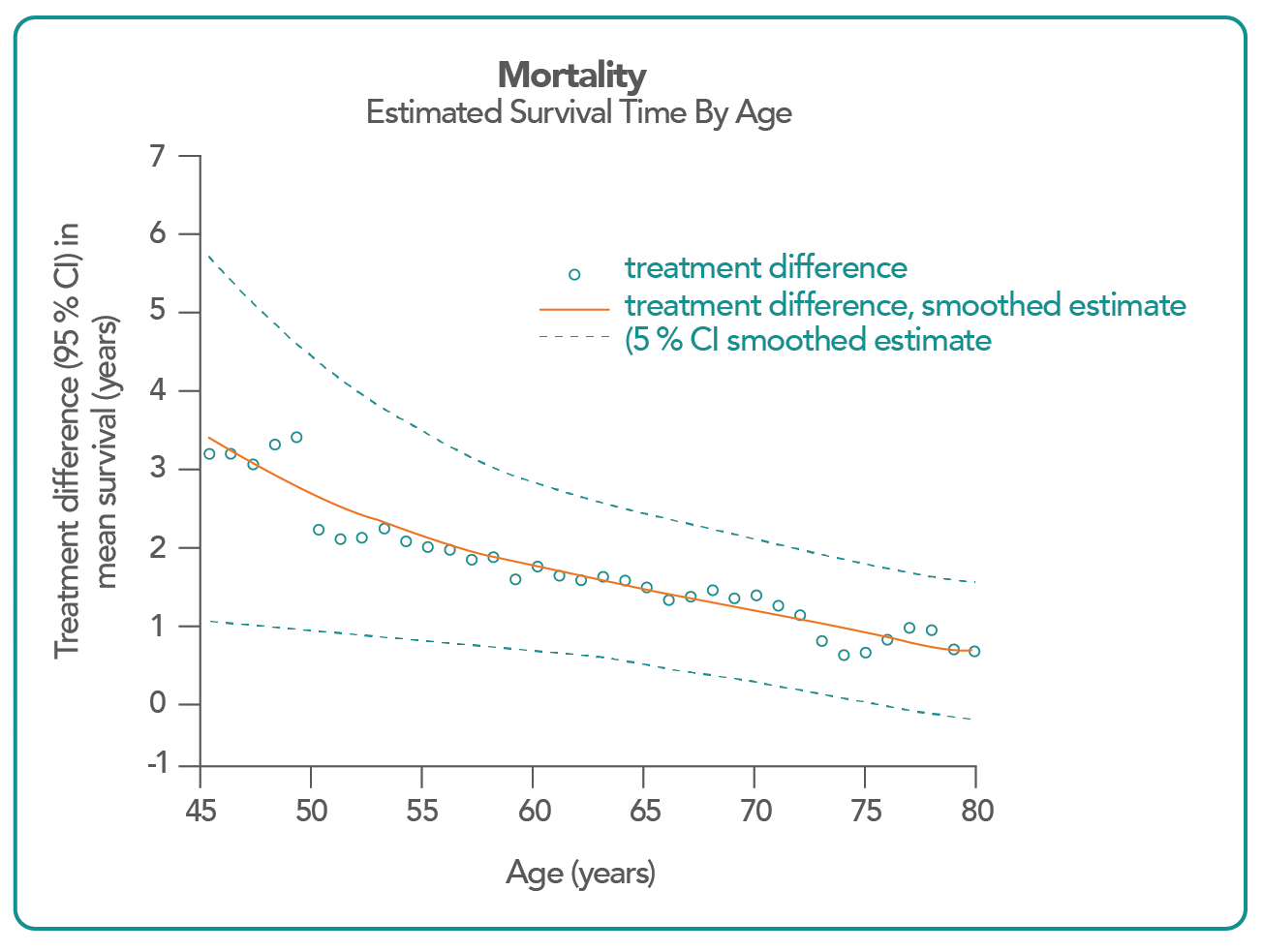
JARDIANCE could add 2.5 years to the life of a 60-year-old patient with type 2 diabetes and cardiovascular disease.10
American Diabetes Association9
For people with type 2 diabetes and established ASCVD who need more glucose-lowering treatment than metformin and lifestyle changes... incorporating one of the SGLT2 inhibitors or GLP-1 receptor agonists that have been shown to reduce cardiovascular events is recommended.
European Society of Cardiology11
Recently, the SGLT2 inhibitor empagliflozin demonstrated significant reductions in CVD death (by 38%) and all-cause mortality (by 32%), as well as hospitalization for HF (by 35%), when compared to standard care, implying that use of an SGLT2 inhibitor should begin very early in the course of management of patients with DM and CVD7.
Heart Failure Association of the European Society of Cardiology12
Empagliflozin should be considered in T2D patients to prevent or delay the onset of HF.
American Diabetes Association and European Association for the Study of Diabetes Consensus Report9
SGLT2 inhibitors or GLP-1 receptor agonists with proven cardiovascular benefit are recommended as part of glycemic management in patients with type 2 diabetes who have established ASCVD12.
American College of Cardiology Expert Consensus Decision Pathway13
With respect to HF events, SGLT2 inhibitors such as Jardiance have demonstrated substantial benefits in reducing hospitilisations for heart failure and CV deaths.13
References: 1. Martín-Timón I, Sevillano-Collantes C, Segura-Galindo A, Del Cañizo-Gómez FJ. Type 2 diabetes and cardiovascular disease: Have all risk factors the same strength? World J Diabetes. 2014;5(4):444-470. doi:10.4239/wjd.v5.i4.444. 2. IDF Diabetes Atlas. [Online] 2022. [Cites 2022 Apr 4];Available from URL: https://diabetesatlas.org/. 3. Jardiance Approved Package Insert. Ingelheim Pharmaceuticals (Pty) Ltd. December 2017. 4. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med 2015;373:2117-28. DOI: 10.1056/NEJMoa1504720. 5. IDF. Diabetes complications.[Online] 2022 May 22. [Cites 2022 Apr 4];Available from URL: https://www.idf.org/aboutdiabetes/complications.html. 6. Levine MJ. Empagliflozin for Type 2 Diabetes Mellitus: An Overview of Phase 3 Clinical Trials. Curr Diabetes Rev. 2017;13(4):405-423. doi:10.2174/1573399812666160613113556. 7. Anker SD. Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients -EMPA-REG OUTCOME. Oral Presentation American Academy of Cardiology. Date Presented:11/17/2019. Date updated 01/13/2020. 8. Data on File. Ingelheim Pharmaceuticals (Pty) Ltd. 9. Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C et al. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD ). Diabetes Care 2018;41:2669–270. 10. Claggett B, Lachin JM, Hantel S, Fitchett D, Inzucchi SE, Woerle HJ et al. Long-term benefits of empaglifloxin on life expectancy in patients with Type 2 diabetes mellitus and established cardiovascular disease. Research Letter. Circulation 2018;138:1599-1601. 11. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice. European Heart Journal (2016) 37, 2315–2381. doi:10.1093/eurheartj/ehw106. 12. Seferovi´c PM, Coats AJS, Ponikowski P, Filippatos G, Huelsmann M. European Society of Cardiology/Heart Failure Association position paper on the role and safety of new glucose-lowering drugs in patients with heart failure. European Journal of Heart Failure.2020; 22: 196–213. 13. Das S, Everett B, Birtcher K, et al. 2020 Expert Consensus Decision Pathway on Novel Therapies for Cardiovascular Risk Reduction in Patients With Type 2 Diabetes. J Am Coll Cardiol. 2020 Sep, 76 (9) 1117–1145. https://doi.org/10.1016/j.jacc.2020.05.037.

S4 SYNJARDY® 5/500 mg: Each film-coated tablet contains empagliflozin 5 mg and metformin HCl 500 mg. Reg. No. 49/21.2/0915. S4 SYNJARDY® 5/850 mg: Each film-coated tablet contains empagliflozin 5 mg and metformin HCl 850 mg. Reg. No. 49/21.2/0916. S4 SYNJARDY® 5/1000 mg: Each film-coated tablet contains empagliflozin 5 mg and metformin HCl 1 000 mg. Reg. No. 49/21.2/0917. S4 SYNJARDY® 12,5/500 mg: Each film-coated tablet contains empagliflozin 12,5 mg and metformin HCl 500 mg. Reg. No. 49/21.2/0918. S4 SYNJARDY® 12,5/850 mg: Each film-coated tablet contains empagliflozin 12,5 mg and metformin HCl 850 mg. Reg. No. 49/21.2/0919.
S4 SYNJARDY® 12,5/1000 mg: Each film-coated tablet contains empagliflozin 12,5 mg and metformin HCl 1 000 mg. Reg. No. 49/21.2/0920. NAMIBIA. NS2 Synjardy 5/500mg. 16.21.2/0210; NS2 SYNJARDY® 5/850 mg. 16.21.2/0211; NS2 SYNJARDY® 5/1000 mg 16.21.2/0212; NS2 SYNJARDY® 12.5/500 mg 16.21.2/0213; NS2 SYNJARDY® 12.5/850 mg 16.21.2/0214; NS2 SYNJARDY® 12.5/1000 mg 16.21.2/0215. BOTSWANA: S2 SYNJARDY® 5/500 mg: Each film-coated tablet contains empagliflozin 5 mg and metformin HCl 500 mg. Reg. No. BOT2203842. S2 SYNJARDY® 5/850 mg: Each film-coated tablet contains empagliflozin 5 mg and metformin HCl 850 mg. Reg. No. BOT2203843. S2 SYNJARDY® 5/1000 mg: Each film-coated tablet contains empagliflozin 5 mg and metformin HCl 1 000 mg. Reg. No. BOT2203844. S2 SYNJARDY® 12,5/500 mg: Each film-coated tablet contains empagliflozin 12,5 mg and metformin HCl 500 mg. Reg. No. BOT2203845. S2 SYNJARDY® 12,5/850 mg: Each film-coated tablet contains empagliflozin 12,5 mg and metformin HCl 850 mg. Reg. No. BOT2203846. S2 SYNJARDY® 12,5/1000 mg: Each film-coated tablet contains empagliflozin 12,5 mg and metformin HCl 1 000 mg. Reg. No. BOT2203847.
For full prescribing information refer to the package insert approved by the Medicines Regulatory Authority Applicant details: Ingelheim Pharmaceuticals (Pty) Ltd, Suite 1, Building 4, 2nd Floor, Waterfall Corporate Campus, 74 Waterfall Drive, Midrand, 2066. Cpy Reg. No. 1966/008618/07. BI Ref. No. PC-ZA-101593 Expiry Date: February 2024.