Life is worth Living

Don’t let IPF stop your patients THERE IS STILL LIFE TO BE LIVED
IN A POOLED ANALYSIS, THE MEDIAN SURVIVAL FOR PATIENTS WITH IPF WAS EXTENDED BY APPROXIMATELY 5 YEARS WITH OFEV® COMPARED TO PLACEBO*†1
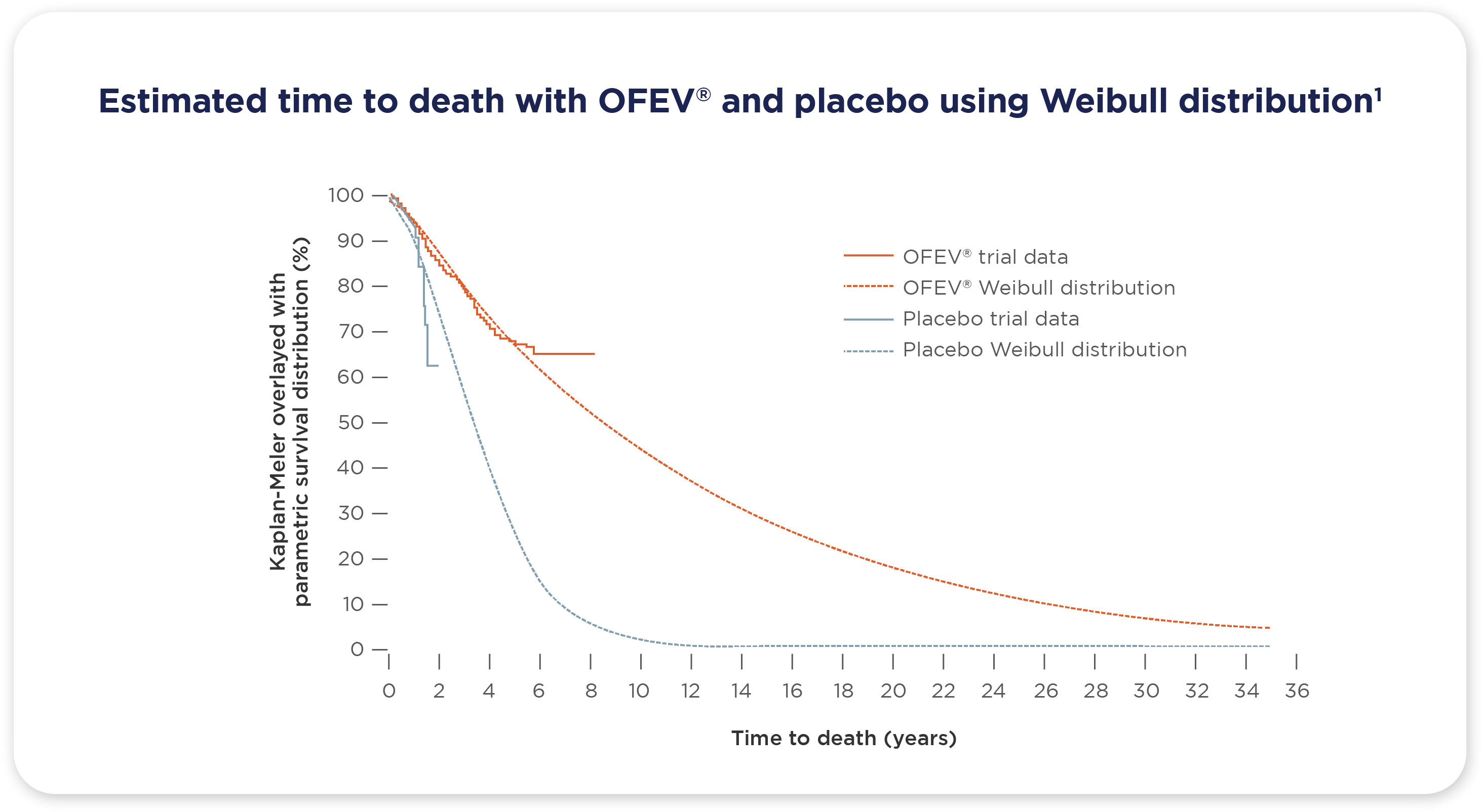
- The pooled data analysis from the clinical trials suggests that OFEV® extends life expectancy in patients with IPF1
- Based on the Weibull distribution, mean survival was estimated as 11.6 years (95% CI 9.6, 14.1) in OFEV®-treated patients and 3.7 years (95% CI 2.5, 5.4) in placebo-treated patients1
IPF is one of the most common and deadly ILDs.2,3
Choose OFEV® for a treatment with demonstrated efficacy4
*Data were pooled from the prospective TOMORROW, INPULSIS®-1 and -2 and INPULSIS-ON® trials, as well as the phase 3b trial (NCT01979952) in patients with IPF. OFEV® population (n=1126): patients treated with ≥1 dose of OFEV® (150 mg twice daily) and patients who switched from blinded placebo to open-label OFEV® in INPULSIS®-ON and the phase 3b trial. Placebo population (n=565): patients treated with ≥1 dose of placebo in the TOMORROW, INPULSIS®-1 and -2 and phase 3b trials.1
†Based on the Weibull distribution. The Weibull model was based on the better fitting statistical model.1
References:
1. Lancaster L, Crestani B, Hernandez P, et al. Safety and survival data in patients with idiopathic pulmonary fibrosis treated with nintedanib: pooled data from six clinical trials. BMJ Open Respir Res. 2019;6(1):e000397. 2. Raghu G, Collard HR, Egan JJ, et al; on behalf of the ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788-824. 3. Oldham JM, Noth I. Idiopathic pulmonary fibrosis: early detection and referral. Respir Med. 2014;108(6):819-829. 4. Richeldi L, du Bois RM, Raghu G, et al; for the INPULSIS® Trial Investigators. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;380(22):2071-2082.
To report any adverse events please contact PV_loca_South Africa@boehringer-ingelheim.com
Should you require any further clinical information about OFEV®, please do not hesitate to contact me at renard.prinsloo@boehringer-ingelheim.com
S4OFEV® 100 mg contains 100 mg nintedanib base (as esilate salt) Reg. No./Nr.: A 52/26/0153.
S4OFEV® 150 mg contains 150 mg nintedanib base (as esilate salt) Reg. No./Nr.: A 52/26/0154.
Applicant details: Ingelheim Pharmaceuticals (Pty) Ltd. Suite 1, Building 4, 2nd Floor, Waterfall Corporate Campus, 74 Waterfall Drive, Midrand, 2066, South Africa. Tel: +27 (011) 348-2400. Cpy. Reg. No. 1966/008618/07. BI Ref. No. PC-ZA-101639. Expiry date: March 2024.