Experience matters
Courage to face IPF head on,
Confidence to treat with OFEV®
OFEV® has a demonstrated treatment effect in reducing FVC decline from treatment initiation, and a safety profile that is sustained over time1-6
OFEV® - A consistent treatment effect over 52 weeks in multiple large, Phase III trials2,3,6
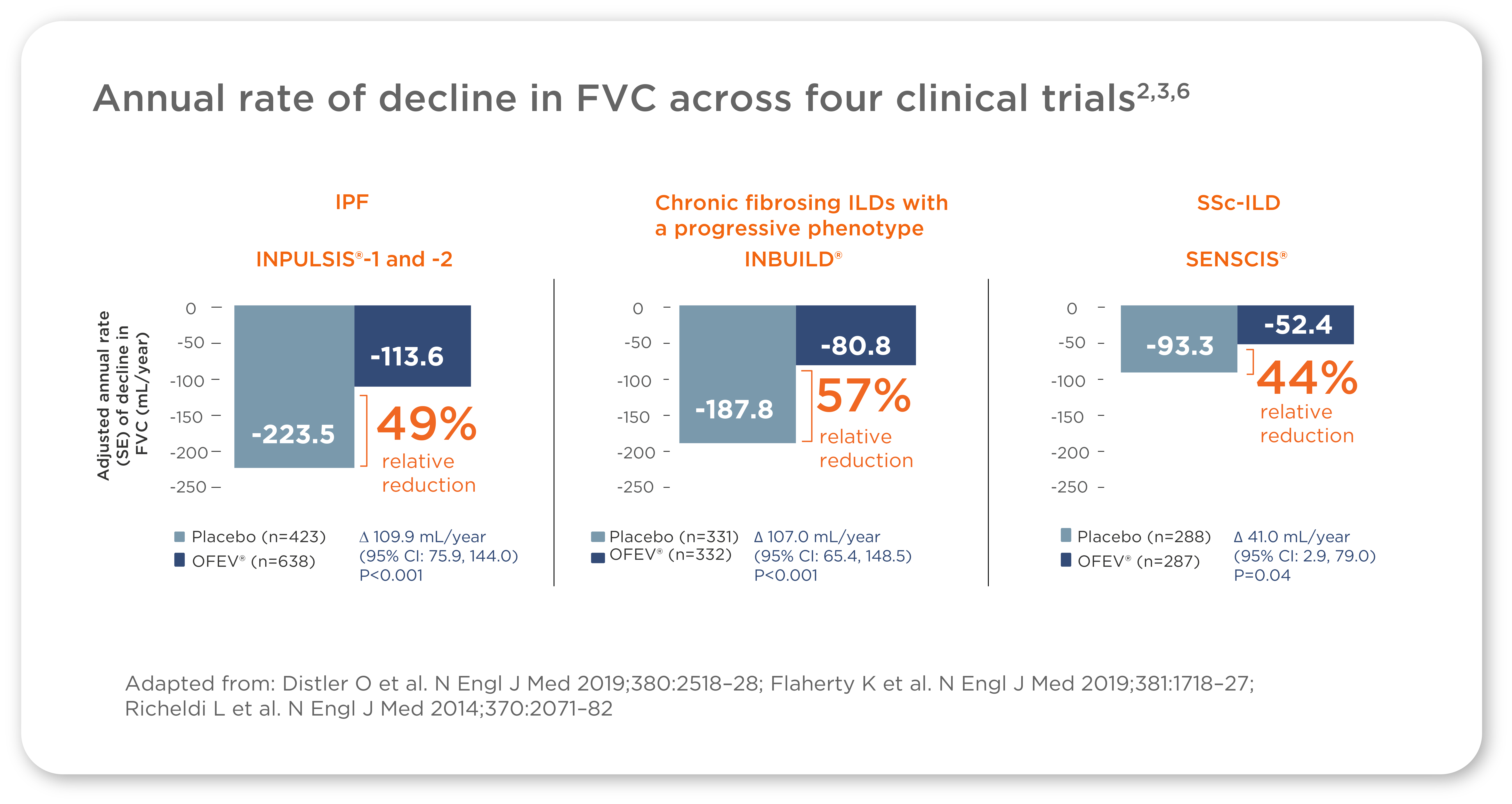
IPF: Idiopathic pulmonary fibrosis; SSc-ILD: Systemic Sclerosis associated interstitial lung disease
The treatment effect on slowing FVC decline was consistent across trials studying patients with IPF, other chronic fibrosing ILDs with a progressive phenotype, and SSc-ILD2,3,6,7
Think OFEV® for a demonstrated treatment effect in slowing FVC decline in patients with IPF, other chronic fibrosing ILDs with a progressive phenotype, and SSc-ILD7-13
An Effect consistent over time….
The INPULSIS®-ON trial confirmed that the efficacy of OFEV® in patients with IPF persists over 4 years1
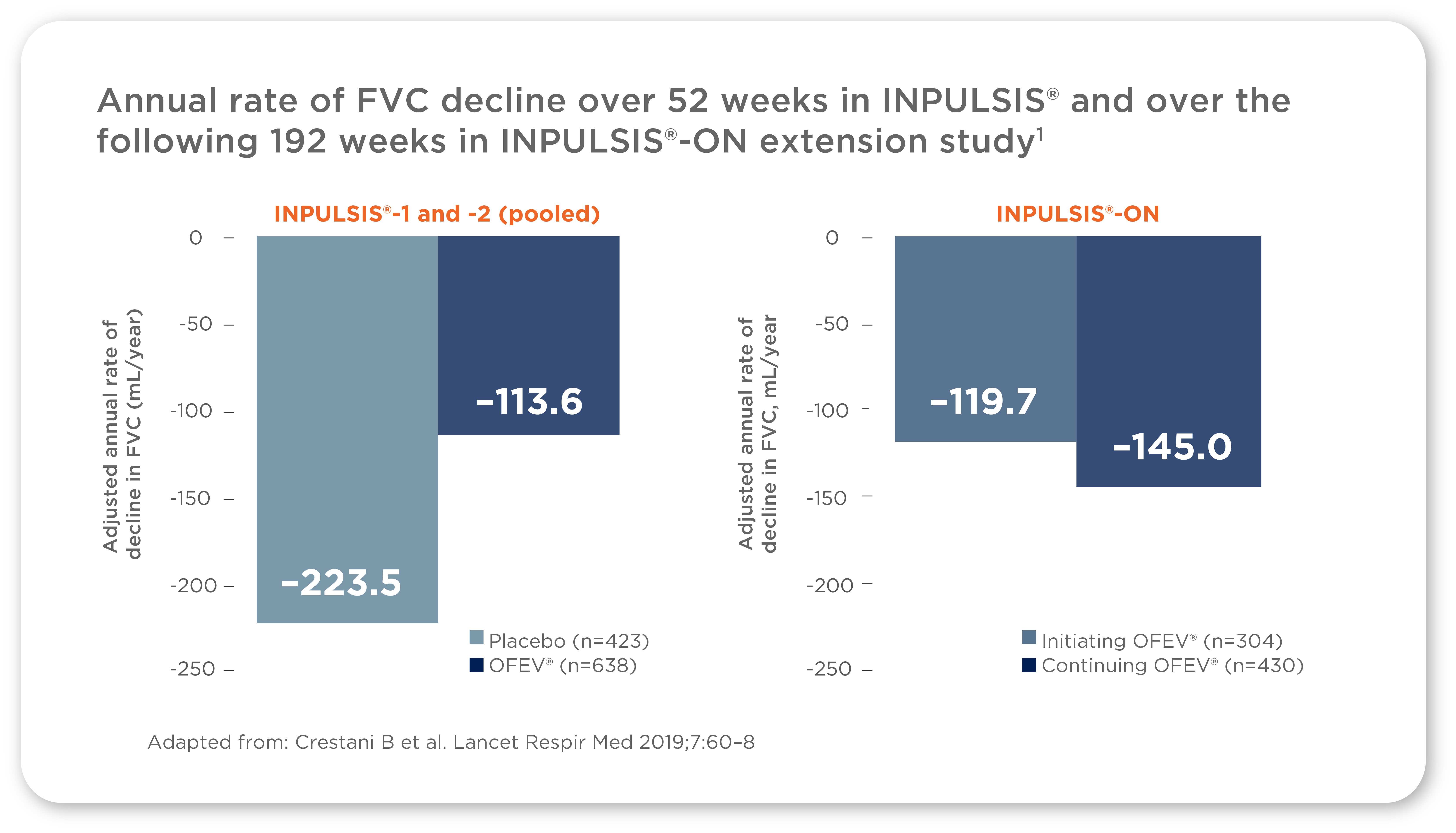
The effect of OFEV® on reducing disease progression in the INPULSIS® trials was consistent over long-term treatment in the INPULSIS®-ON open-label extension trial*†1
*Per protocol, the off-treatment period between INPULSIS® and INPULSIS®-ON could be between 4 and 12 weeks1
†The differences in FVC decline in INPULSIS®-ON between patients who received OFEV® and placebo in the preceding INPULSIS® trials were not considered to be clinically meaningful, particularly when put into perspective with the FVC decline observed in placebo-treated patients in INPULSIS® (-223.5 mL/year) and the minimal clinically important difference in FVC, which is believed to be 2–6% predicted (a difference of at least 75–80 mL)1,14
Additionally, long-term OFEV® treatment (up to 68.3 months) had a manageable safety and tolerability profile, with no new safety signals identified1
Consider OFEV® for sustained efficacy and safety in patients with IPF over 4 years1,6,10
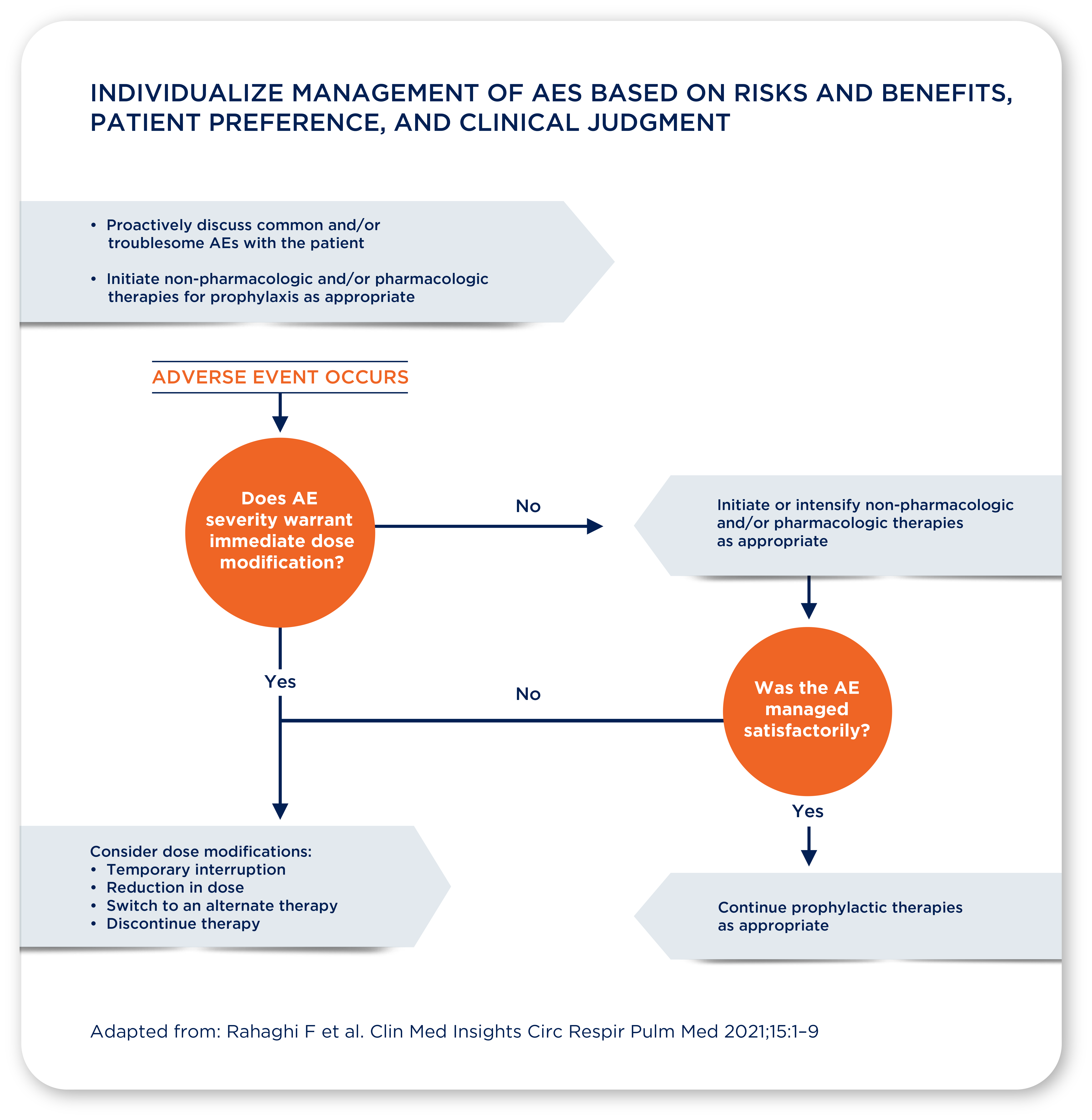
Manage your patients, so they can remain on treatment:
In clinical trials across all of OFEV®’s indications, AE management helped patients to remain on therapy3,15,16
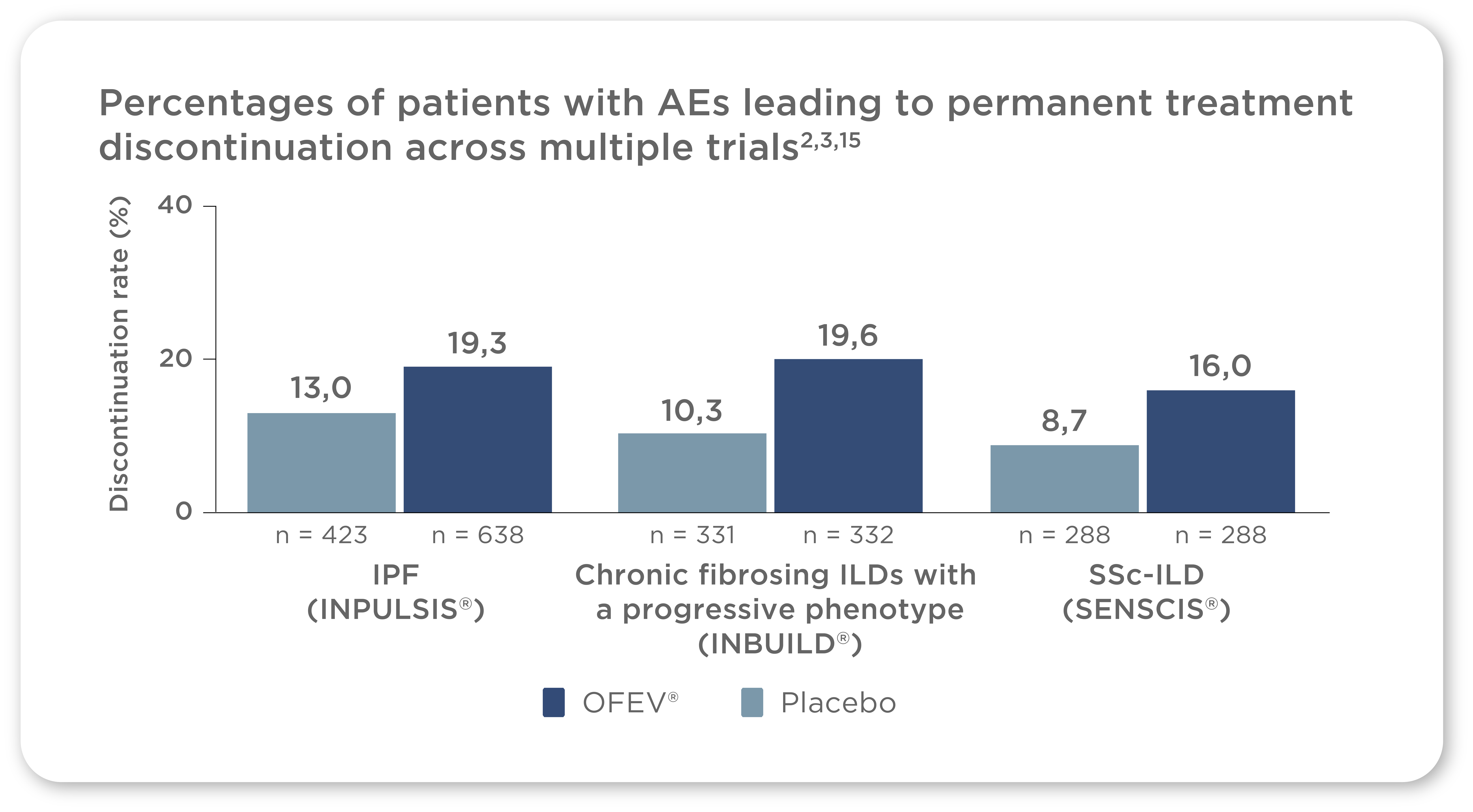
Across the INPULSIS®, SENSCIS®, and INBUILD® trials, AEs resulted in OFEV® discontinuation in 16.0–19.6% of patients,2,3,15 suggesting that OFEV® treatment can be effectively managed in most patients
Most patients were able to remain on treatment in each trial2,3,15
Management of common Side effects
GI Side effects
Supportive medications7
Antidiarrheals, such as loperamide
Antiemetic therapy
Dose adjustment7
Temporary treatment interruption or dose reduction (100 mg twice daily) if symptomatic treatment is ineffective If a patient does not tolerate 100 mg twice daily, treatment with OFEV® should be discontinued
Dietary changes7,17
Adequate hydration at first sign of diarrhea Avoidance of dried fruits with high pectin content Use of the BRAT (bananas, rice, applesauce, toast) diet
DECREASED APPETITE AND WEIGHT LOSS
- Decreased appetite can be managed using quality food/protein intake, high-protein nutritional drinks and more frequent meals. Weight loss can be managed using increased calorie intake, a dietary schedule of three meals per day with snacks in between, and high-protein nutritional drinks17
- Dose modification (reduction or interruption) should be initiated if a patient with decreased appetite loses >4.5 kg (>10 lbs)17
- Dose modification (reduction or interruption) should be considered in patients with persistent weight loss17
LIVER ENZYMES
- Liver enzyme elevations have been reported with OFEV® treatment; in most cases, these were reversible on dose reduction or treatment interruption*7
- Dose reduction or interruption of OFEV® treatment plus close monitoring is recommended if AST/ALT >3 x ULN occurs7
- OFEV® treatment should be permanently discontinued if liver enzyme elevations occur in association with clinical signs and symptoms of liver injury7