Life is worth Living

Don’t let IPF stop your patients THERE IS STILL LIFE TO BE LIVED
Expect Consistent Safety with OFEV®1
The safety profile of OFEV® (nintedanib) observed in the INPULSIS® trials was sustained in the INPULSIS®-ON trial*1
Exposure to OFEV® in INPULSIS® and INPULSIS®-ON (n=430)1§
12Months
Minimum exposure1
45Months
Median exposure1
68Months
Maximum exposure1
- INPULSIS®-ON was an open-label extension trial that assessed the long-term efficacy and safety of OFEV® in 734 patients with IPF1
§ 430 patients had received Nintedanib in INPULSIS and continued Nintedanib in INPULSIS-ON.1
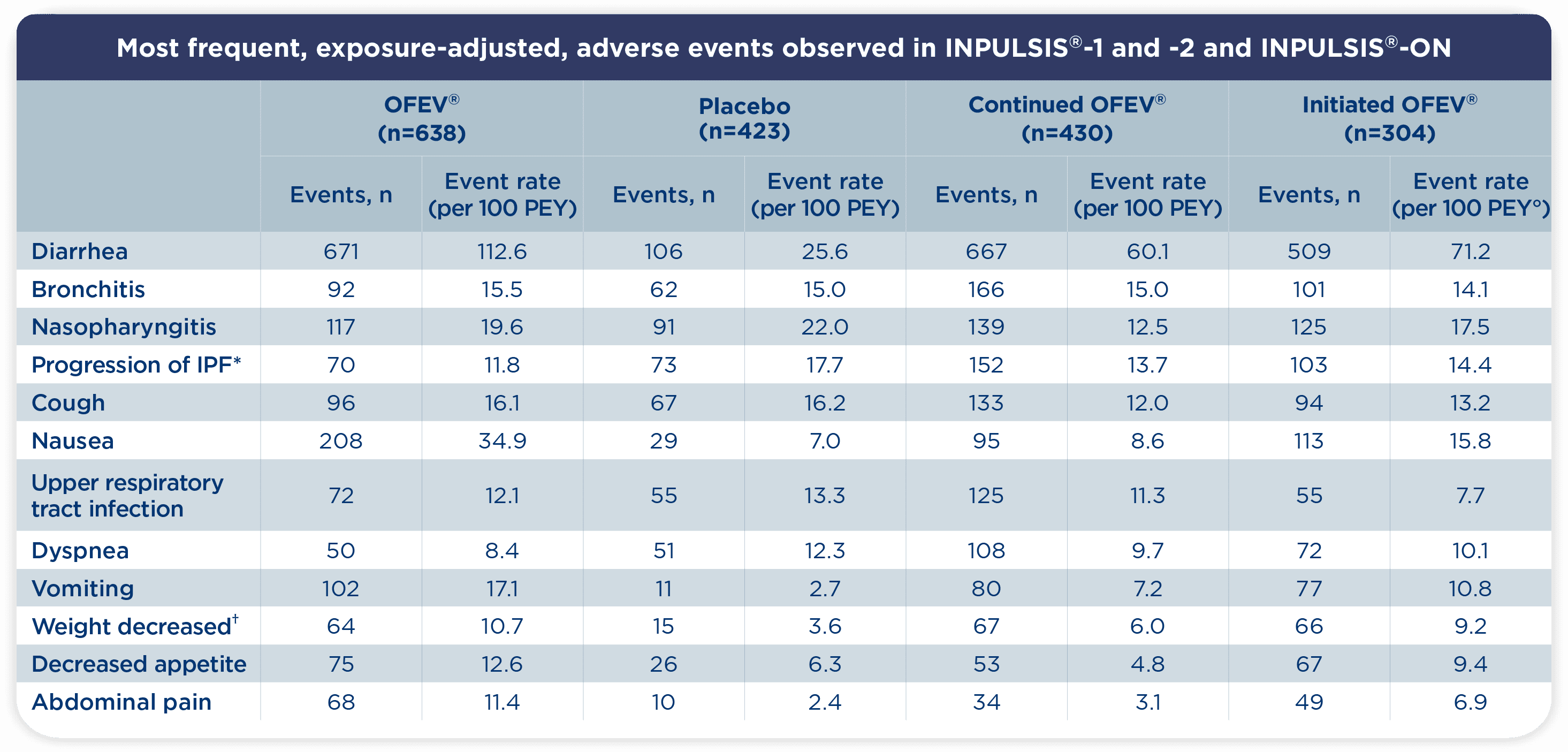
° PEY: Patient Exposure Years. Adverse events with an event rate >10 per 100 patient exposure–years in any of the groups shown are listed1

- Consistent with INPULSIS®, diarrhea was the most frequently reported adverse event with OFEV® in INPULSIS®-ON1

68Months
Continued treatment with OFEV® for up to 68 months had a manageable safety and tolerability profile†1

There were no new safety signals identified1
The safety profile of OFEV® was shown to be consistent with the INPULSIS® trials‡1
†Median total exposure for those treated with OFEV® in both the INPULSIS trials and the INPULSIS-ON trial extension was 44.7 months and the maximum total exposure was 68.3 months. [Crestani 2019]
‡Rates of major adverse cardiovascular events, myocardial infarction, and bleeding in patients were similar to those observed in patients who received placebo during the INPULSIS trials, suggesting there is no increased risk of these events with long-term use of OFEV®. [Crestani 2019]
Choose OFEV® to provide patients with IPF a treatment with a demonstrated long-term safety profile1
References:
1. Crestani B, Huggins JT, Kaye M, et al. Long-term safety and tolerability of nintedanib in patients with idiopathic pulmonary fibrosis: results from the open-label extension study, INPULSIS-ON. Lancet Respir Med. 2019;7(1):60-68
To report any adverse events please contact PV_loca_South Africa@boehringer-ingelheim.com
Should you require any further clinical information about OFEV®, please do not hesitate to contact me at renard.prinsloo@boehringer-ingelheim.com
S4OFEV® 100 mg contains 100 mg nintedanib base (as esilate salt) Reg. No./Nr.: A 52/26/0153.
S4OFEV® 150 mg contains 150 mg nintedanib base (as esilate salt) Reg. No./Nr.: A 52/26/0154.
For full prescribing information refer to the professional information approved by the Regulatory Authority.
For full prescribing information refer to the professional information approved by the Regulatory Authority.
Applicant details: Ingelheim Pharmaceuticals (Pty) Ltd. Suite 1, Building 4, 2nd Floor, Waterfall Corporate Campus, 74 Waterfall Drive, Midrand, 2066, South Africa. Tel: +27 (011) 348-2400. Cpy. Reg. No. 1966/008618/07. BI Ref. No. PC-ZA-101640. Expiry date: March 2024.