Life is worth Living

Don’t let IPF stop your patients THERE IS STILL LIFE TO BE LIVED
A BETTER QUALITY OF LIFE
OFEV® (nintedanib) reduced the worsening of cough and dyspnea in patients with chronic fibrosing ILDs with a progressive phenotype1,2
The burden of progressive pulmonary fibrosis in ILD can substantially reduce a patient's health-related quality of life3
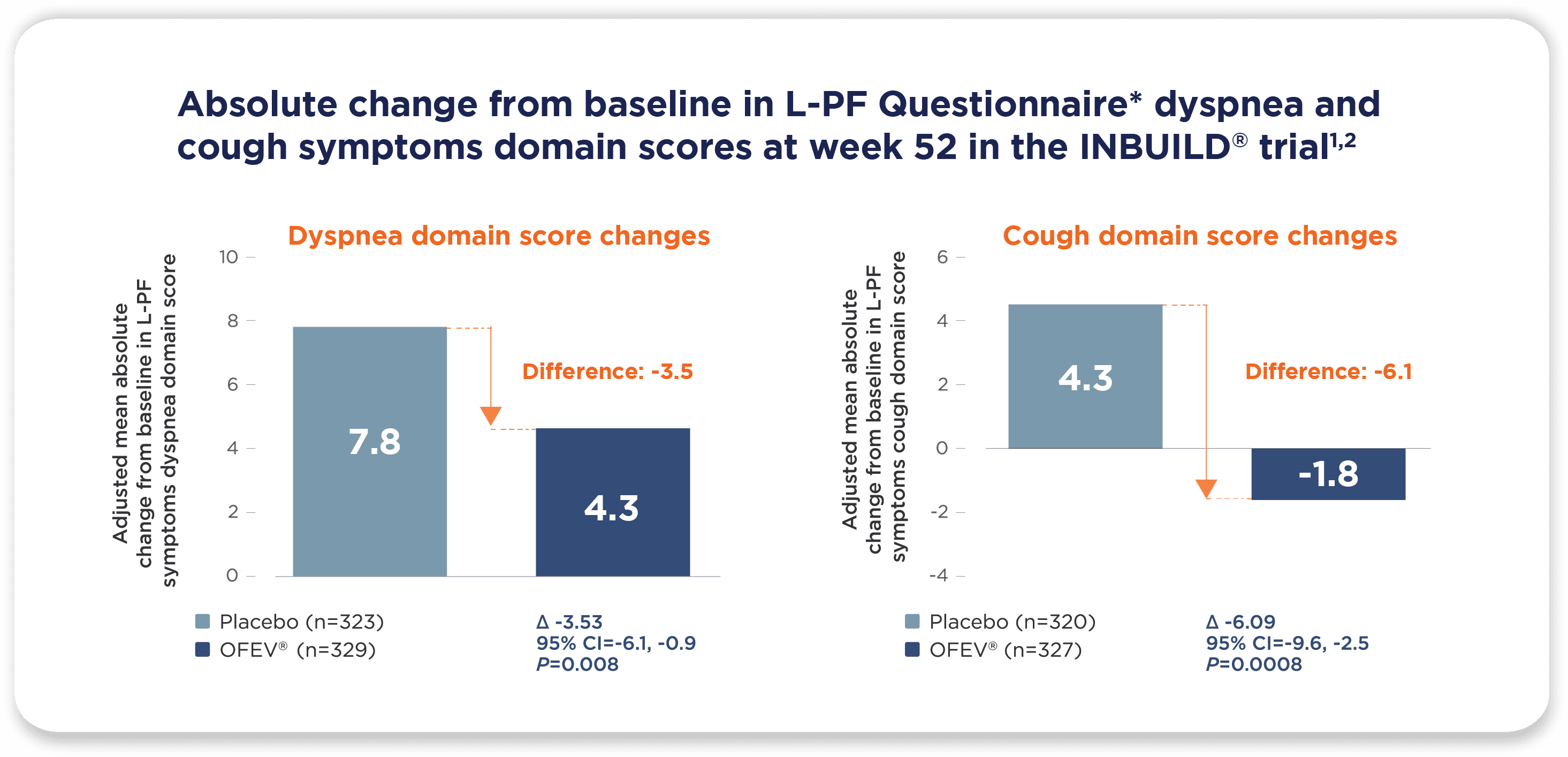
*The L-PF Questionnaire was developed to assess quality-of-life impact using input from patients with pulmonary fibrosis. The L-PF symptoms module consists of domains. Scores across 3 domains (dyspnea, cough, fatigue) range from 0 to 100. Higher scores represent greater symptomatic impairment and worse health status.
CONSIDER OFEV® FOR A BETTER QUALITY OF LIFE1,2 BECAUSE LIFE IS WORTH LIVING

References:
1. OFEV® Professional information leaflet; Ingelheim Pharmaceuticals (Pty) Ltd 10 December 2021. 2. Swigris JS, Richeldi L, et al; Effects of nintedanib on dyspnea, cough and quality of life in patients with progressive fibrosinginterstitial lung diseases (ILDs): findings from the INBUILD® trial. Am J Respir Crit Care Med 2020;201:A2754. 3. Flaherty KR, Wells AU, Cottin V, et al. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med. 2019;381(18):1718-1727
To report any adverse events please contact PV_loca_South Africa@boehringer-ingelheim.com
Should you require any further clinical information about OFEV®, please do not hesitate to contact me at renard.prinsloo@boehringer-ingelheim.com
S4OFEV® 100 mg contains 100 mg nintedanib base (as esilate salt) Reg. No./Nr.: A 52/26/0153.
S4OFEV® 150 mg contains 150 mg nintedanib base (as esilate salt) Reg. No./Nr.: A 52/26/0154.
For full prescribing information refer to the professional information approved by the Regulatory Authority.
For full prescribing information refer to the professional information approved by the Regulatory Authority.
Applicant details: Ingelheim Pharmaceuticals (Pty) Ltd. Suite 1, Building 4, 2nd Floor, Waterfall Corporate Campus, 74 Waterfall Drive, Midrand, 2066, South Africa. Tel: +27 (011) 348-2400. Cpy. Reg. No. 1966/008618/07. BI Ref. No. PC-ZA-101641. Expiry date: March 2024.